What is a Mineral?

A mineral is a naturally occurring, inorganic solid with an orderly crystalline structure and definite chemical composition.
Minerals must have these characteristics...
- Naturally occurring- Forms naturally in Earth. Man made gems are not minerals.
- Solid Substance- Are solid within the temperature ranges that are normal for Earth's surface, not a glass nor liquid.
- Orderly Crystalline Structure-The atoms or ions of the minerals are arranged in an orderly and repetitive pattern.
- Definite Chemical Composition- Most minerals are chemical compounds made of two or more elements. Only a few are native elements such as gold and silver. It will always have the same chemical formula.
- Generally Considered Inorganic- Most minerals are inorganic crystalline solids found in nature. Which means not organic or living, compounds. The word "generally" is used because there is an exception (i.e. calcium, carbonate, which comes from coral reefs or shells from marine animals.)
How do Minerals Form?
Minerals form nearly everywhere on Earth under different conditions. There are four major processes by which minerals form: crystallization from magma, precipitation, changes in pressure and temperature, and formation from hydrothermal solutions.
*Crystallization from Magma

Magma is molten rock, it forms deep inside Earth. When magma cools, elements join together to form minerals like feldspar, quartz, muscovite, and hornblende.
*Precipitation

Not from the sky!! This certain type of precipitation happens when dissolved minerals in water form a solid once the water has evaporated (i.e. saltwater will leave behind the salt and the water evaporates, but you can still see the salt when the water is present). Like this limestone cave image caused by precipitation.
*Pressure & Temperature

Under extreme pressure and temperature, existing minerals become unstable and are forced to rearrange its atoms to form a new mineral. (recrystallize). Example is muscovite like the image here.
*Hydrothermal Solutions

A hydrothermal solution is a very hot mixture of water and dissolved substances. The temperatures are between 100 degrees Celsius and 300 degrees Celsius. When the solution touches existing minerals a chemical reaction happens and a new mineral is made. Sometimes when it cools, some elements come together and form minerals like bornite and pyrite.
Mineral Groups
- Silicates- Silicon and oxygen combine to form a structure called the silicone-oxygen tetrahedron. Ex. Quartz and Feldspar
- Carbonates- Are minerals that obtain the element carbon, oxygen and one or more other metallic elements. Ex. Calcite and Dolomite
- Oxide- Are minerals that contain oxygen and one or more other element which is usually metal. Ex. Rutile and Corundum
- Sulfates and Sulfides- Are minerals that contain the element sulfur.
Ex. Gypsum and Galena - Halides-Are minerals that contain a halogen ion plus one or more other elements. Ex. Halite (table salt) and Fluorite
- Native elements- Are a group of mineral that exist in relativity pure form.
Ex. Gold (Au) and Copper (Cu)
Properties of Minerals
- Color- some amount of elements give the mineral its certain color, but it is NOT a great indicator for minerals!
- Streak- is the color of a mineral in its powdered form
- Luster-is used to describe how light is reflected from the surface of a mineral
- Crystal Form-is the visible expression of a mineral's internal arrangement of atoms
- Hardness-is a measure of the resistance of a mineral to it being scratched
- Cleavage-is the tendency of a mineral to cleave, or break along flat, even surfaces
- Fracture- are minerals that do not show cleavage. It is also the uneven breakage of a mineral.
- Density-is a property of all matter that is the ratio of an object's mass to its volume.
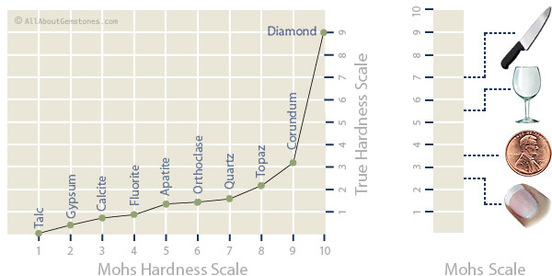
Mohs Hardness Scale
The Mohs scale was devised by a Geman mineralogist, Friedrich Mohs in 1812. The Mohs scale measures the hardness of minerals. It characterizes the scratch resistance of various minerals through the ability of a harder material to scratch a softer material. Therefore one which is talc is the softest up to the number 10 which is diamond is the hardest mineral. On the left is the hardness of objects used to test an objects hardness with.