Elements
What is an Element?

An element is a substance that cannot be broken down into simpler substances by chemical or physical means. Many common elements are metals such as copper, gold, and silver.
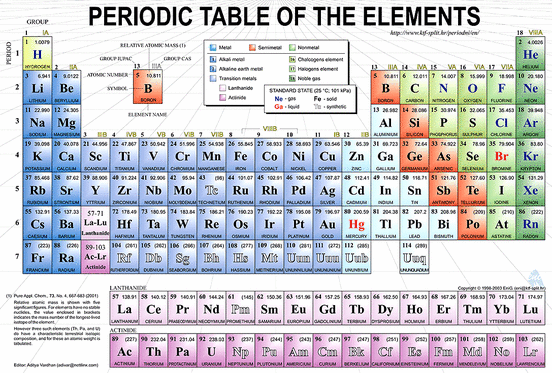
There are more than 112 known elements, and new elements continue to be discovered. About 92 of these elements are naturally occurring, the other are man-made. The elements have been organized by their properties in a document called the periodic table. (Picture above) You see from the picture that the name of each element is represented by a symbol consisting of one, two, or three letters.
Terms dealing with the Periodic Table
* Periods- rows on the periodic table
* Groups- are the colomns on the periodic table (there are 18 groups)
* Atomic number - equal to the number of protrons to the nucleus or electrons in the neutral state of an atom of an element
* Mass number- the sum of the number of neutrons and protons in an atomic nucleus
* Groups- are the colomns on the periodic table (there are 18 groups)
* Atomic number - equal to the number of protrons to the nucleus or electrons in the neutral state of an atom of an element
* Mass number- the sum of the number of neutrons and protons in an atomic nucleus
Atoms
What is an Atom?
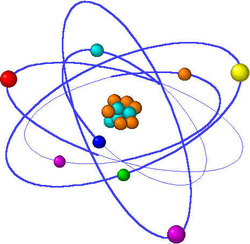
An atom is the smallest particle of matter that contains the characteristics of an element.
The center of an atom is called the nucleus. The nucleus contains protrons and neutrons. Electrons are the particles that surround the atom's nucleus.
The center of an atom is called the nucleus. The nucleus contains protrons and neutrons. Electrons are the particles that surround the atom's nucleus.
Protrons and Neutrons
Protrons is a stable particle with positive charge than neutrons have a neutral charge. The number of protons in the nucleus of an atom is called the atomic number.
Electron
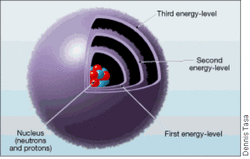
Electrons is a particle with a negative charge. electron has a mass of about 1/1836 the mass of a proton or a neutron. Electrons move about the nucleus so quickly they create a sphereshaped negative zone. Electrons are on the ouside of the nucleus like the picture above which is called an energy level or shells. The shells each have a certain amount of electrons.
Isotopes
Atoms with the same number of protons but different numbers of neutrons are isotopes of an element.
Compounds
A compound is a substance that consists of two or more elements that are chemically combined in specific proportions.
Types of Bonds
Paragraph.